Dilution Definition Literature . a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the concept of dilution is first explained using a mass balance equation that was generated based on the fact that the mass. Concentration is the removal of solvent, which. dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution.
from exoaaybok.blob.core.windows.net
dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is a process used to lower the concentration of the original solution by adding more solvent. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the concept of dilution is first explained using a mass balance equation that was generated based on the fact that the mass.
Dilution Method Microbiology at Harry Hausman blog
Dilution Definition Literature Concentration is the removal of solvent, which. dilution is a process used to lower the concentration of the original solution by adding more solvent. Concentration is the removal of solvent, which. Concentration is the removal of solvent, which. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the concept of dilution is first explained using a mass balance equation that was generated based on the fact that the mass. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution.
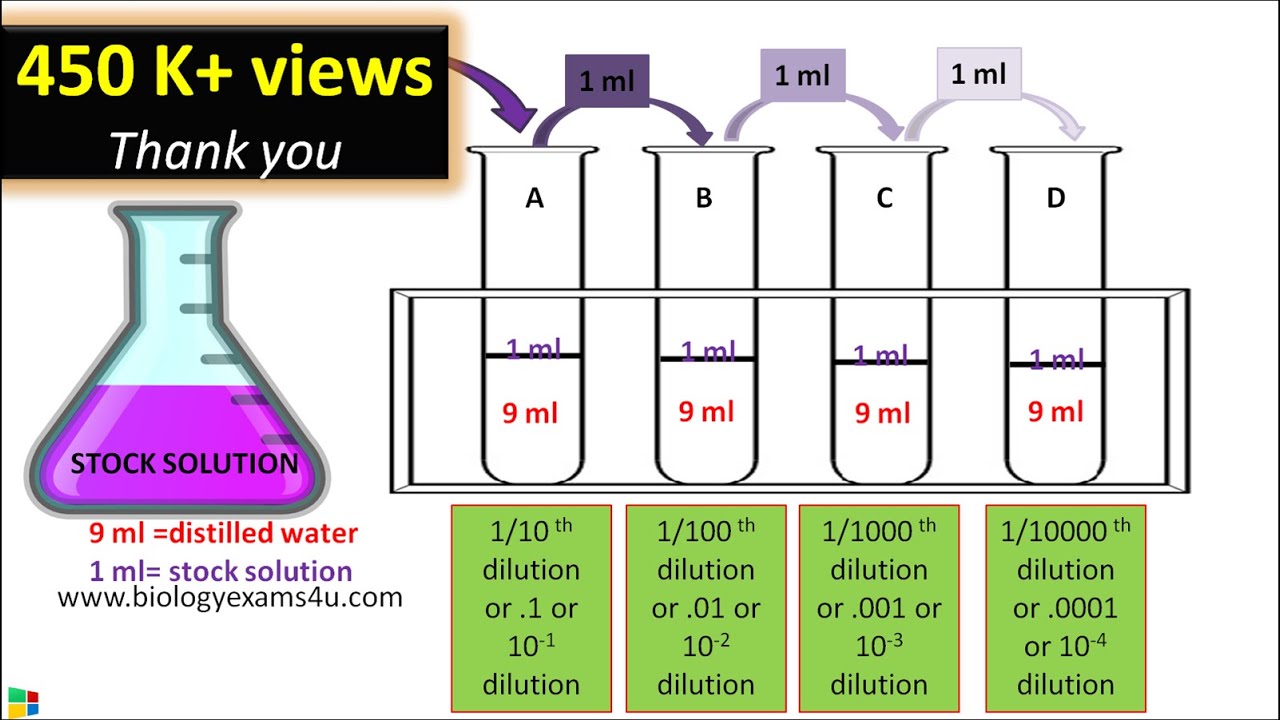
From dxorsyqnq.blob.core.windows.net
Dilution Adjective Definition at Robert Gonzales blog Dilution Definition Literature dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the addition. Dilution Definition Literature.
From dxopdbmbn.blob.core.windows.net
Dilution Symbol at Andrea Bloom blog Dilution Definition Literature dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is a process used to lower the concentration of the original solution by adding more solvent. . Dilution Definition Literature.
From biologyideas.com
Serial Dilution in Microbiology Definition, Formula, Procedure and Dilution Definition Literature a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution refers to the process. Dilution Definition Literature.
From prabhakarpk.blogspot.com
Serial Dilution Definition, Formula, Calculator, Procedure, Uses Dilution Definition Literature a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is a process used to lower the concentration of the original solution by adding. Dilution Definition Literature.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Definition Literature Concentration is the removal of solvent, which. the concept of dilution is first explained using a mass balance equation that was generated based on the fact that the mass. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. dilution is a process used to lower. Dilution Definition Literature.
From exoaaybok.blob.core.windows.net
Dilution Method Microbiology at Harry Hausman blog Dilution Definition Literature a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the concept of dilution is first explained using a mass balance equation that was generated based on the fact that the mass.. Dilution Definition Literature.
From fabrikbrands.com
What Is Brand Dilution? Brand Dilution Definition With Examples Dilution Definition Literature Concentration is the removal of solvent, which. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the. Dilution Definition Literature.
From exoyaaeul.blob.core.windows.net
Dilution Definition In A Sentence at Tomas Branson blog Dilution Definition Literature dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. Concentration is the removal of solvent, which. the concept of dilution is first explained using a mass balance equation that was generated based on the fact that the mass. a dilute solution is one. Dilution Definition Literature.
From www.aquaportail.com
Dilution définition et explications AquaPortail Dilution Definition Literature dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. the concept of dilution is first explained using a mass balance equation that. Dilution Definition Literature.
From studylib.net
Dilutions MCHS Science Dilution Definition Literature dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. the concept of dilution is first explained using a mass balance equation that was generated based on the fact that the mass. dilution is the addition of solvent, which decreases the concentration of the solute in. Dilution Definition Literature.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Dilution Definition Literature dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the concept of dilution is first explained using a mass balance equation that was generated based on the fact that. Dilution Definition Literature.
From adropofthis.blogspot.com
A Drop of This The Solution for Dilution Dilution Definition Literature Concentration is the removal of solvent, which. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is a process used to lower the concentration of. Dilution Definition Literature.
From slideplayer.com
Proportional Relationships ppt download Dilution Definition Literature dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the. Dilution Definition Literature.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution Definition Literature dilution is a process used to lower the concentration of the original solution by adding more solvent. the concept of dilution is first explained using a mass balance equation that was generated based on the fact that the mass. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution. Dilution Definition Literature.
From www.scribd.com
Dilution Calculations Examples and Practice Problems for Diluting Dilution Definition Literature Concentration is the removal of solvent, which. Concentration is the removal of solvent, which. dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. dilution is the addition of. Dilution Definition Literature.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Definition Literature dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is a process used to lower the concentration of the original solution by adding more solvent. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. . Dilution Definition Literature.
From www.thoughtco.com
What Is the Definition of Dilute? Dilution Definition Literature dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the. Dilution Definition Literature.
From www.slideshare.net
Serial dilution Dilution Definition Literature dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the concept of dilution is first explained using a mass balance equation that was generated based on the fact that the mass. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the. Dilution Definition Literature.